
By Hendre’ Barnard, Training and Marketing Manager
Distillique Beverage (Pty) Ltd.
https://distillique.co.za
Click <Here> to see more of Hendre’s articles.
One of the catch phrases in the Home Distilling Community is “Low and Slow”.
This refers to “Low Flame” or “Low Heat”, and the resulting “Slow” Distillation Process.
This article addresses and explains this concept by describing the effect that the boiling rate in a batch boiler (i.e. not a continuous still) has on the composition of fractions during the distilling run, and what changes in Distillate Flow Rate or the Rate of Heat Addition has on product quality.
The effect can be summarised as follows:
When boiling very, very slowly, you would be able to predict (i.e. know) what comes out of the still
over time and be able to select fractions that you prefer to have.
When boiling fast, you will not know what comes out of the still and have to be happy with what you get.
We can illustrate, and explain, these graphically.
In the diagrams below, the following is applicable:
- The height of the bar indicates a relative distillate flow rate, i.e. drops per second, millilitres per minute or litre per hour
- The width of the bar indicates the relative time for the distillation. (i.e. minutes or hours)
- The area of the bar (height x width) equals the total yield or volume of distillate recovered
To simplify matters, we assume that the flow rate remains the same throughout the entire distillation run, with the same heat input (i.e. kW energy) over time.
This is strictly speaking not true, as the flowrate will gradually decrease over time because more energy is required to evaporate low alcohol mashes, than for high alcohol mashes (because the heat of vaporisation for water is much higher than the heat of vaporisation of alcohol).
As distillation is the removal of ethanol from fermentation, the alcohol percentage in the fermentation therefore decreases over time, hence more energy is required over time to maintain a constant flowrate.
In the figures below, we also work on a fixed amount of mash to be distilled for all the examples.
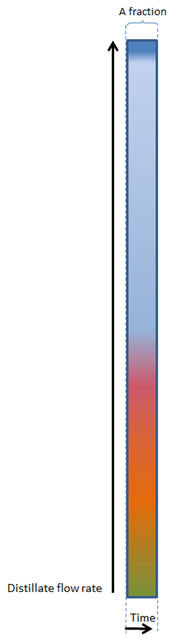
For a very, very slow boiling distillation, the following figure will be applicable:
First the heads (the majority of the most volatile components such as methanol and acetone) will come through, then the hearts (ethanol) and then the tails (heavier alcohols / fusel oils / aromatic components).
These main fractions are indicated with the colour bands.
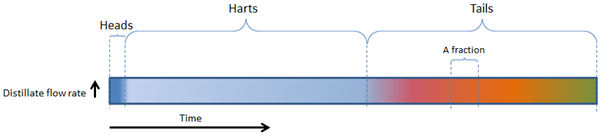
1. SLOW BOILING
We can see that the heads, hearts and tails are fairly easy to distinguish, as well as various fractions/components within the tails (indicated by the pink, red, orange and yellow fractions within the tails).
PLEASE NOTE – A Fraction can also refer to a Cut made during the run. As illustrated in Figure 1, it may refer to a specific part of the distillate yield that has been removed. Either a shot glass, a cup, a jam jar, a litre, a bucket, etc.
However, in the example above, with a very, very slow rate of boiling, little vapour is formed and as a result, little vapour can be condensed to become distillate, and this gives a relatively low distillation flow rate.
The result thereof is that the distillation runs over a relative long period of time.
An intuitive conclusion might be that we would be able to reduce the distillation time in half by doubling the heat input.
This intuitive conclusion is hundred percent correct.
Double the heat input WILL half the distillation time (if we ignore slightly higher heat losses for the moment).
However, something else also changes which is not necessarily intuitive.
In Figure 2 (below), note that the distinction between the fractions (colour bands – can also refer to individual cuts) become more difficult and that the fractions at a specific point, changes in composition.
As Figures 2, 3, 4 and 5 (below) show, the vertical lines between heads, hearts and tails (and the various factions within the tails) changes from perfectly vertical, to slightly slanted, to very slanted and eventually to horizontal in figure 5.
During very, very slow boiling (Figure 1) a fraction (or cut) will be fairly constant in composition.
In fact, if we look at a very small fraction (or cut), it will only contain one very specific component (or type of alcohol) found in the fermentation.
In Figure 2, 3, 4 and 5, the composition of even a small fraction, progressively becomes more and more complex and in Figure 5, even the smallest fraction will contain all the components found in a mash.
2. A LITTLE FASTER BOILING
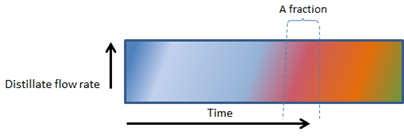
3. EVEN FASTER BOILING
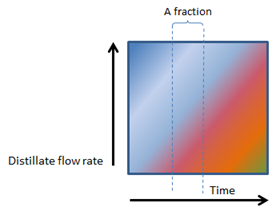
4. FAST BOILING
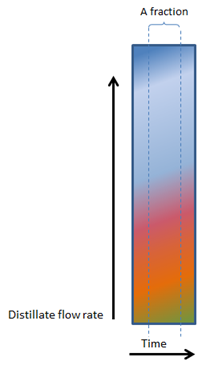
5. VERY, VERY FAST BOILING
The figures above illustrate the effects of the boiling rate (i.e. heat input) on the composition of fractions from the resulting distillate.
As a result, we can make the following deductions:
- The greater the rate of heat addition, the faster the boil
- The faster the boil, the greater the rate of evaporation, the greater the rate of condensation (assuming of course the condenser is capable of cooling all the vapour)
- The greater the rate of distillate flow (i.e. production rate), the shorter the amount of time required to distill a given amount of fermentation
- The greater the rate of heat addition, the faster the fermentation boils, but the less distinct the fractions become
The implication of all this is that we can make clearer cuts while doing a slow batch distillation, and this allows us to decide which fractions we would like to retain in our final product.
Inversely, if we run our still fast, we cannot differentiate between the various fractions as clearly and we will find that the components start to “smear” across one another.
NOTE:
From the start of a run, to the end of the distillation run, the temperature will gradually increase over time as the composition of the mash changes over time.
Initially, with a relative high alcohol percentage, the boiling point will be lower. As the alcohol content becomes less, the boiling temperature rises.
If you measure, record and correlate the temperatures with the various fractions, you would be able to work on temperatures alone during consecutive distilling runs (for that particular kind of mash at the same ambient air pressure / altitude above sea level)
Once temperatures have been correlated with the fractions it is possible to compile a theoretical distilling heat input profile to boil SLOW when necessary and faster to save time without the fractions smearing.
A simple distilling profile might look like this:
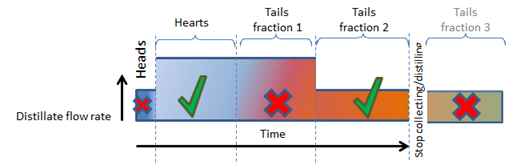
- Start with a slow boil until the heads have been cleanly separated.
- Then double up on the boiling rate while ethanol (the heart) comes off.
- After the hearts we have to discard the first Tail Fraction but can maintain the higher boiling rate.
- To clearly capture the second Tail Fraction, we again have to lower the boiling rate to clearly capture it.
- After the second Tail Fraction has been captured, we can actually stop distilling because the rest of the tails may not be wanted (in this particular example) and it is needless to continue (unless you are a Commercial Distiller where Excise Rules and Regulations require the recovery of all alcohol from fermentations).
Following the above reasoning, it is clear that we can cut out all congeners, with almost no ethanol left in it.
This also implies that it is needless to take the feints (unused heads and tails) and add it to future washes to extract more ethanol from it IF it boiled very slowly in the first run.
Now please note that I did say THEORETICALLY, because in practice, playing around with the Rate of Heat Addition, and therefore the Boiling Rate during the distillation run will cause Surge Boiling, and Surge Boiling leads – once again – to smearing of components.
For the best quality distillate, you should keep the still running Low and Slow throughout the entire run, giving you clean separation between the fractions, and allowing you to take off cuts or fractions in small quantities throughout the run.

If you do this properly the first time you distill a given recipe, and you record the temperature at which the first drop fell into each shot glass, jam jar or whatever measurement you are using to take these cuts, and you then evaluate each of those cuts separately for quality (indicated by the Green Ticks for Good and Red Crosses for Bad) you can compile a graph like this:
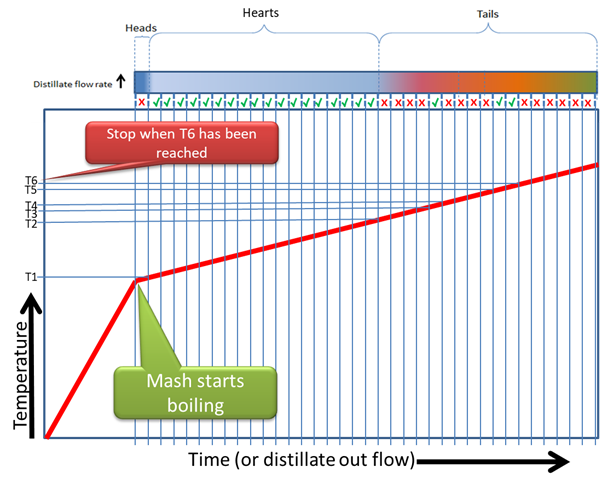
Now we have a plot, that tells us which components (Good or Bad) came out at which temperature.
This allows us to complete the run the next time in exactly the same way and get the exact same quality product out at the end, by deciding which Cuts or Fractions to keep or discard, based on changing temperature.
For instance, I may now know my Fermentation will start boiling at 78 degrees Celsius, and from 78 degrees to 81 degrees was my heads. From 81 degrees to 89 degrees is my Hearts cut, but from 89 to 91 degrees is bad flavours. From 91 to 92 degrees is good flavours, but from 92 to 94 degrees is bad flavours. From 94 to 95 degrees was good, but after 95 degrees everything else is bad and can be discarded.
Now I can distill according to changing temperatures, the same way every time.
But this only works if you use the same fermentation recipe (raw material, yeast, fermentation temperature, etc), the same still (because of reflux), in the same location (because of height above sea level).
Change a single variable, and you have to start all over again.
Hope this helps you make good product, and Keep on Distilling!


